Compliance without the Cortisol.
Meet ERMA Evaluate® — the AI platform eliminating inefficiency from promotional review. Designed specifically for life sciences organizations, ERMA replaces manual claim mapping, fragmented workflows, and reactive compliance checks with intelligent automation. ERMA accurately assigns references, verifies claims against approved labeling, and ensures every asset aligns with current FDA guidance. The platform streamlines collaboration across medical, legal, regulatory, and commercial teams — reducing review cycles and accelerating time to market.
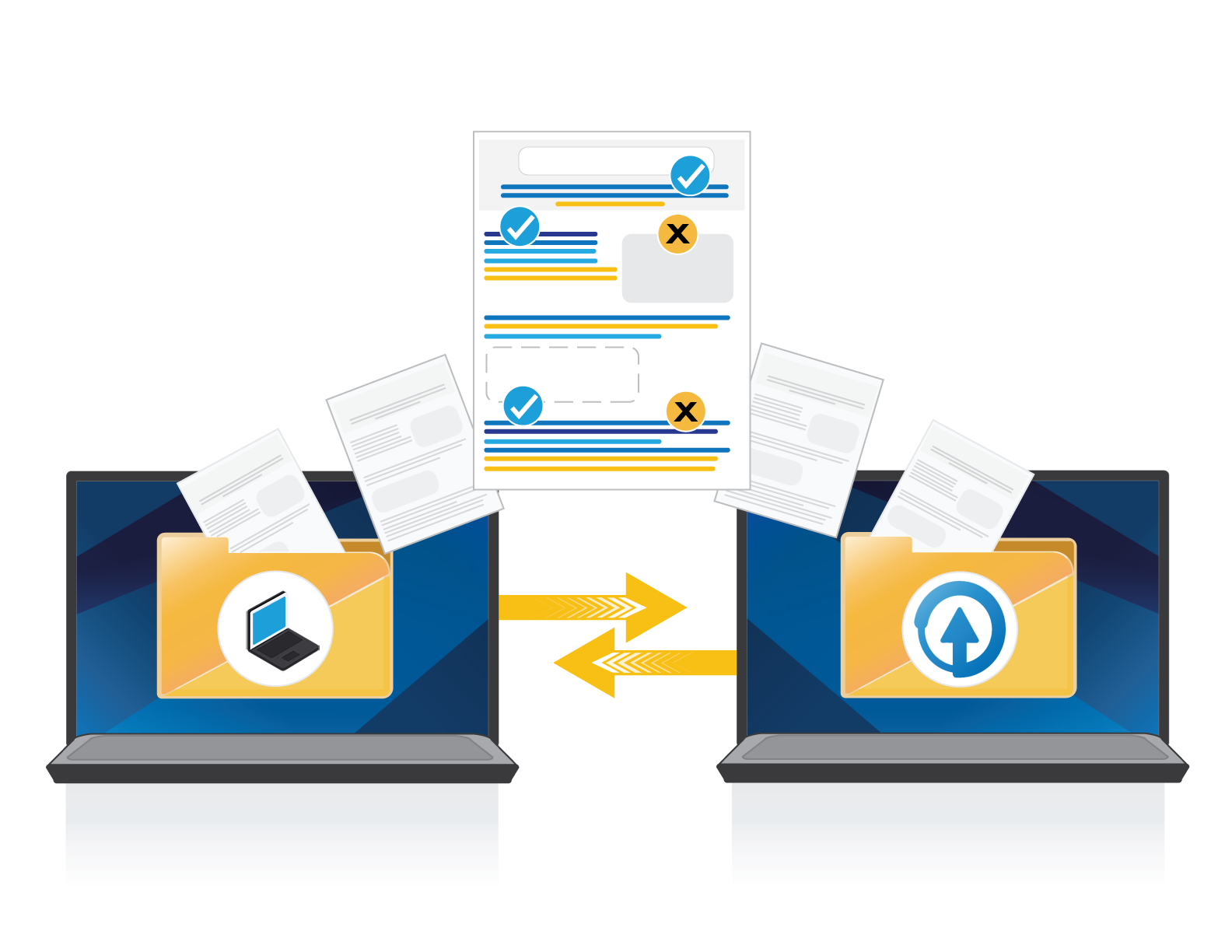
Migrate Existing WorkFlows
Migrate existing archived projects, clinical references, claims, and approved pieces to ERMA Systems™ fast and efficiently.
Solutions for all Industries
in Life Sciences
Pharmaceutical and Biotechnology
Medical Devices and Instruments
Gene Therapy and Emerging Therapies
Animal
Sciences
Using bespoke AI capabilities to improve compliance and process
equates to a 50% reduction in MLR review cycle times.
Improve accuracy of project submissions
Our AI Powered capabilities identify specific claims and references that are appropriate for use. This reduces time needed for reviewers.
Reduce MLR Review Cycle Times
Identify medical, legal, and regulatory resources that have bandwidth to support. Assign a status to the project so reviewers know the priority.
Get Materials to Market Faster
Improved accuracy of project submissions, reduction in review cycles, and automated preparation of 2253 documents lets you hit the ground running.
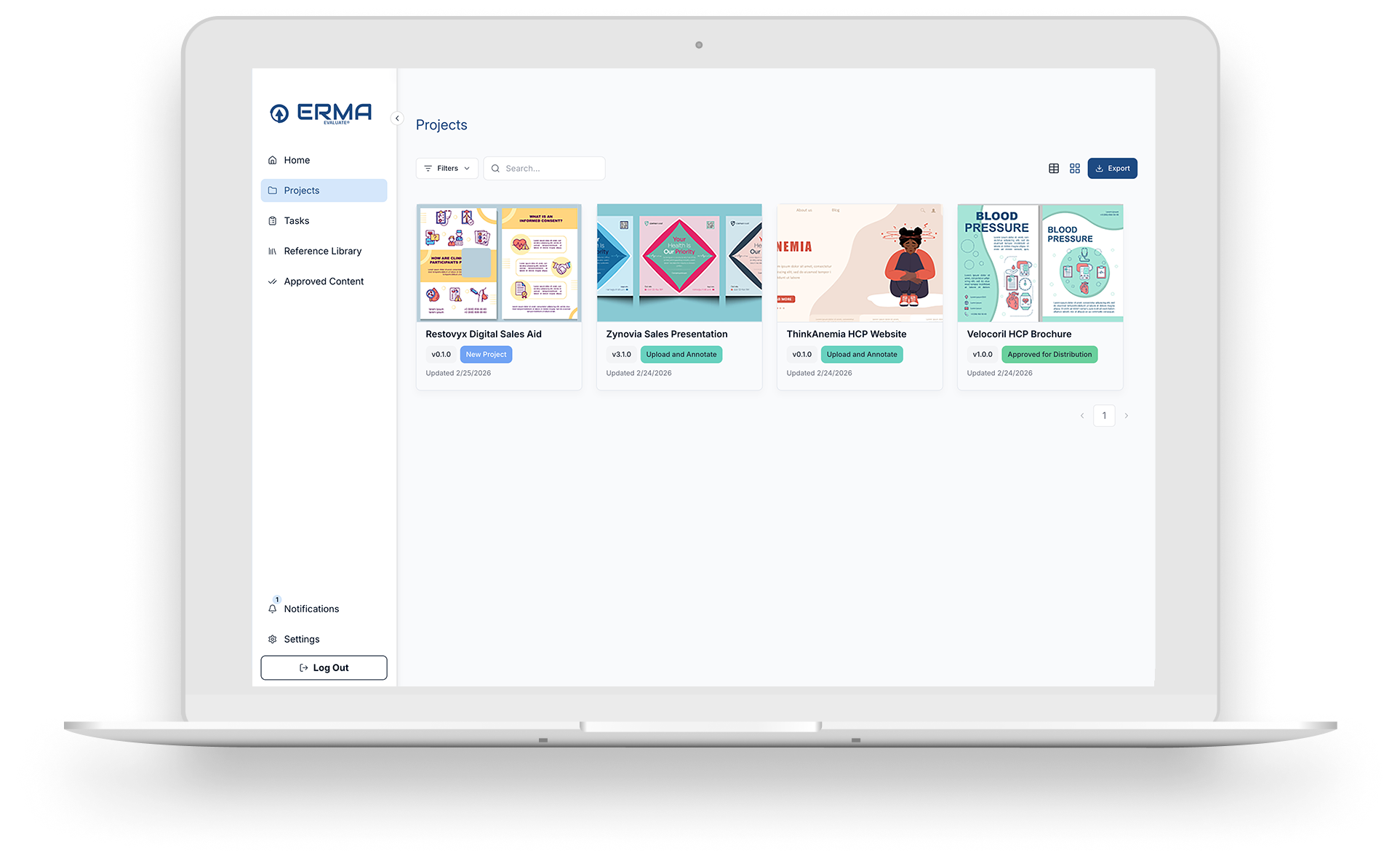
ERMA Comparison
News
ERMA Systems Launches Q1 AI Pilot with Life Science Innovators, Welcomes Brandon Schlette to Advisory Board
ERMA Systems™, the AI-powered compliance platform redefining how life-sciences companies manage medical, legal, and regulatory (MLR/PRC) review, announced today that multiple organizations will participate in its Q1 2026 pilot program—an 8–10-week evaluation designed to validate ERMA’s technology in real-world pharmaceutical workflows.
August 26, 2025ERMA Systems Welcomes Israel Aker, PharmD, RPh, Thermo Fisher Scientific to Advisory Board to Strengthen Medical Affairs Strategy
ERMA Systems, the AI-powered SaaS company transforming how Life Sciences organizations manage their Promotional and Medical Review Committee (PRC/MRC) processes, proudly announces the appointment of Israel Aker, PharmD, RPh, to its Advisory Board.
May 14, 2025ERMA Systems Appoints Christine Hendrick, Director of Commercial Services, to Advisory Board
ERMA Systems, a leading provider of intelligent MLR (Medical, Legal, and Regulatory) management solutions for the life sciences sector, is pleased to announce the appointment of Christine Hendrick, currently Director of Commercial Services at Acadia Pharmaceuticals Inc., to its Advisory Board.
February 26, 2025MAAST DIGITAL Unveils AI-Powered ERMA Evaluate® : A Revolutionary Platform Set to Transform the Traditional Medical, Legal and Regulatory Review Process for Life Science Companies
MAAST DIGITAL is proud to announce ERMA Systems its flagship product, ERMA Evaluate®. This groundbreaking solution is set to redefine the Medical, Legal, and Regulatory (MLR) review process by integrating advanced AI, cutting-edge project management tools, and seamless content creation capabilities into a comprehensive platform.
