An AI-powered Medical, Legal, Regulatory (MLR) platform that streamlines compliance, optimizes processes, and stays ahead of evolving FDA regulations
Meet ERMA, an AI-powered platform designed to simplify the MLR review process for life sciences companies. Leveraging bespoke AI, ERMA accurately assigns references, ensures compliance with approved product claims, streamlines workflows, and generates additional approved promotional materials—enabling teams to bring content to market faster and more efficiently. Continuously trained on the latest FDA regulations, ERMA ensures every review stays current and compliant.
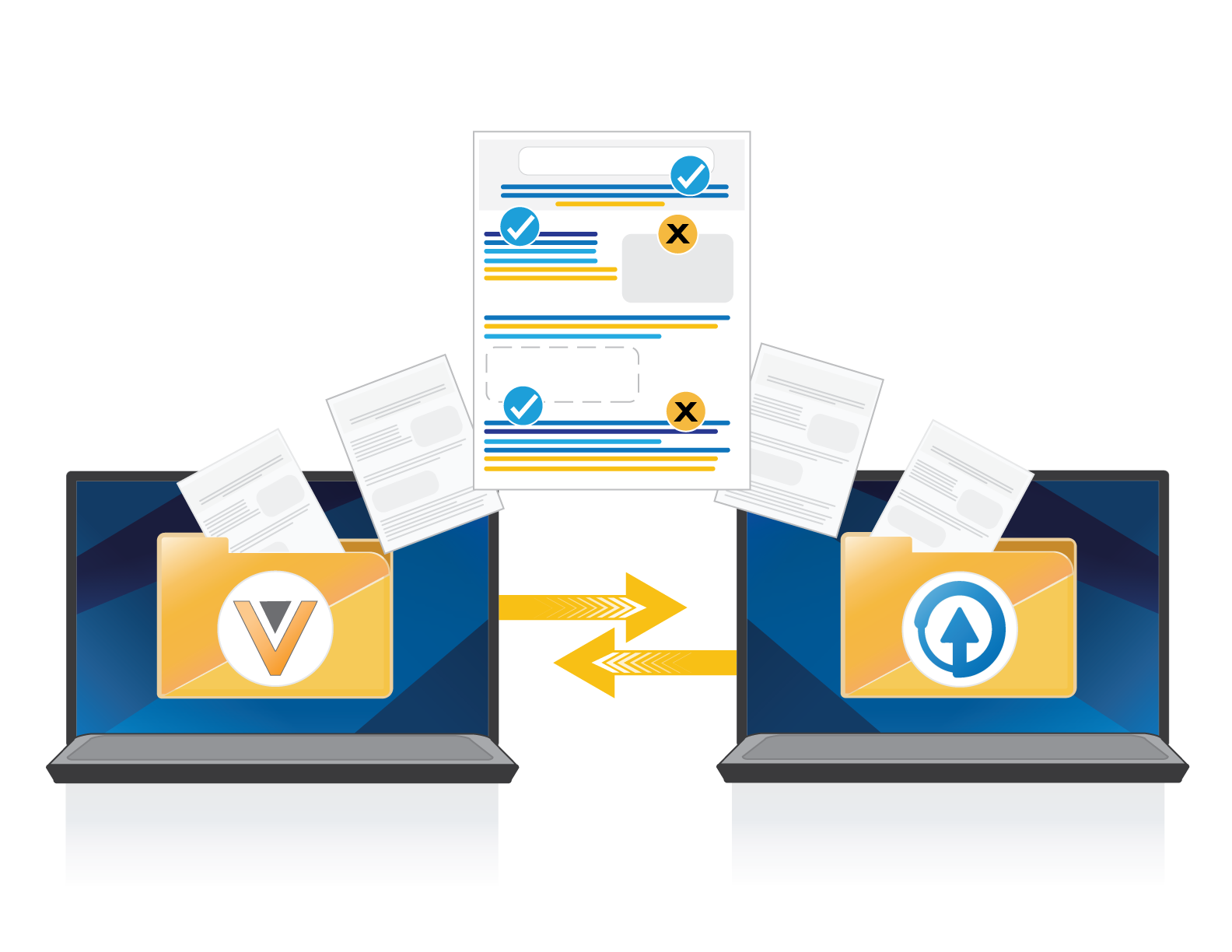
Migrate Existing WorkFlows from Veeva PromoMats
Migrate existing archived projects, clinical references, claims, and approved pieces to ERMA Systems™ fast and efficiently.
Solutions for all Industries
in Life Sciences
Pharmaceutical and Biotechnology
Medical Devices and Instruments
Gene Therapy and Emerging Therapies
Animal
Sciences
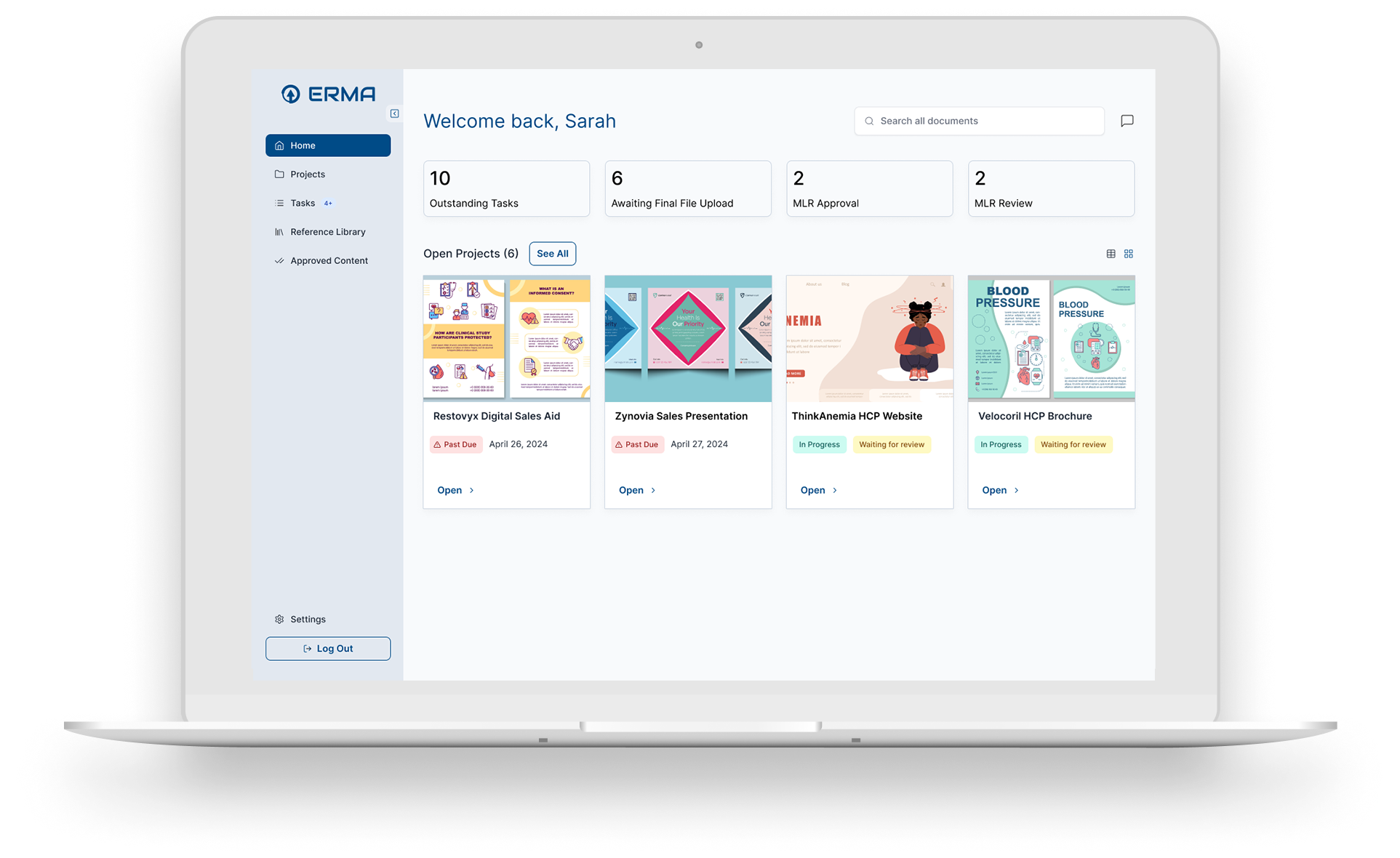
Using bespoke AI capabilities to improve compliance and process
equates to a 50% reduction in MLR review cycle times.
Improve accuracy of project submissions
Our AI Powered capabilities identify specific claims and references that are appropriate for use. This reduces time needed for reviewers.
Reduce MLR Review Cycle Times
Identify medical, legal, and regulatory resources that have bandwidth to support. Assign a status to the project so reviewers know the priority.
Get Materials to Market Faster
Improved accuracy of project submissions, reduction in review cycles, and automated preparation of 2253 documents lets you hit the ground running.
